WDDTY Issue 236 Jun./Jul. 2025
Case
No. 19471,
Published
in the New
England Journal of Medicine,
describes
an eight-year-old Irish American boy admitted
to
Massachusetts General Hospital in 1933.
He had
begun vomiting four days earlier, but clearly, he had been ill for some time.
There was
so little tissue beneath his sallow skin that he appeared emaciated. The lenses
of both of his eyes, which seemed extraordinarily bright blue against his
yellow complexion, had detached. His mother thought his poor vision accounted
for his slow learning—he had attended kindergarten, but his school had refused
him entry to upper grades.
His
tongue protruded to the left, and tests suggested he may have suffered a
stroke. His breathing was shallow and irregular, his blood pressure sky-high at
150/92. Four days later, he died of a stroke.
Looking
at images from the autopsy report, doctors reviewing the puzzling case wondered
how a boy of eight could have the completely clogged carotid artery of an
elderly man.1
More than
30 years later, in 1965, a nine-year-old Irish American girl was seen at
Massachusetts General Hospital for slow mental development. Her eye lens was
detached, and she had other symptoms of homocystinuria, a rare genetic disease
that had recently been discovered in Belfast, Northern Ireland. It was
characterized by high levels of the amino acid homocysteine in the urine and
blood plasma. Laboratory tests confirmed soaring levels of homocysteine in her
blood. Taking her family history, the doctors learned that her uncle had died
in his childhood of a similar condition. He was, in fact, the eight-year-old
who had mysteriously died of a stroke three decades earlier.
Rare cases lead to a
new theory
About this time, Dr
Kilmer McCully, a young pathology instructor at Harvard, became intrigued by
these rare homocystinuria autopsy reports, including that of a two-month-old
baby boy who had died of heart failure. The child’s arteries looked exactly
like what would be found in very elderly patient with advanced vascular
disease.
Drawing on other
research in animals showing that homocysteine is a toxic intermediary in
metabolism, McCully developed a theory for the pathology of arteriosclerosis:
What if homocysteine is the primary driver of arterial damage, and what if that
damage, which is so obvious and devastating in these rare young cases, is also
occurring more subtly in the population at large, among people with high
homocysteine levels in their blood?
McCully didn’t
dismiss the importance of cholesterol in vascular disease, but he suspected
homocysteine was the underlying cause of arteriosclerosis, triggering
cholesterol’s oxidation, buildup and artery-ravaging inflammatory effects. In
other words, homocysteine metabolism and biochemistry determine the more
downstream effects of cholesterol.
|
|
|
|||
|
What causes high
homocysteine The following are
common factors causing homocysteine |
|
||||
|
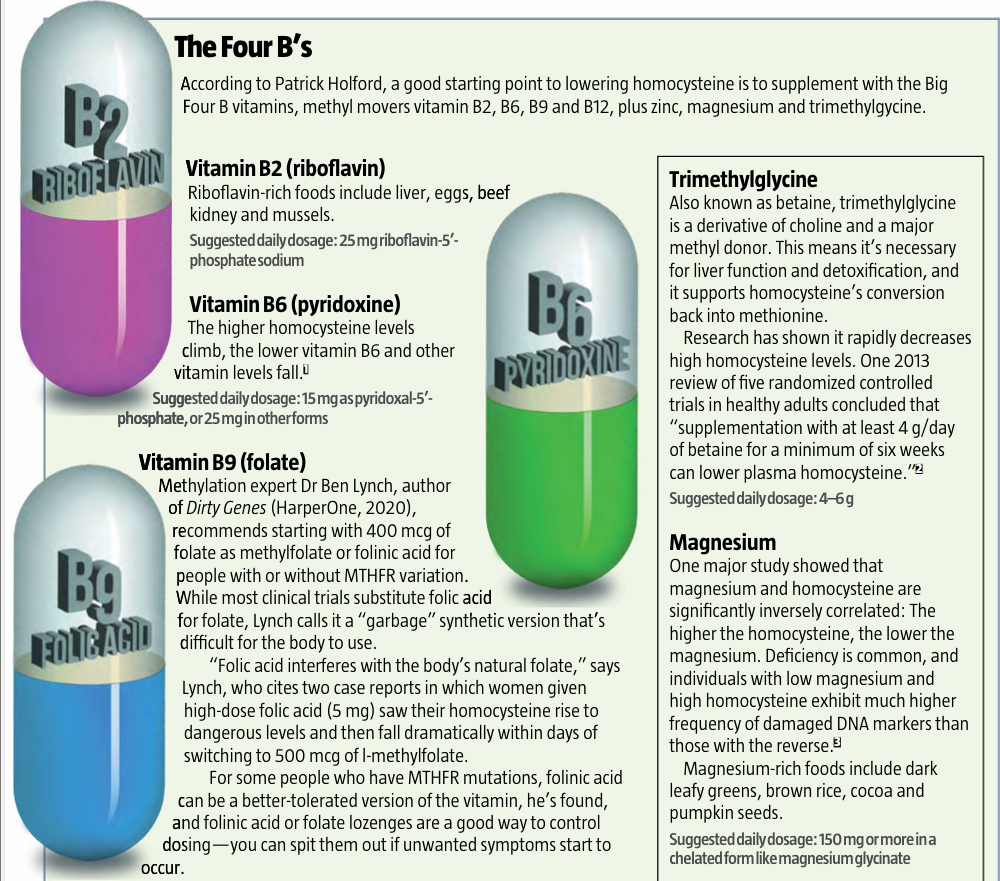
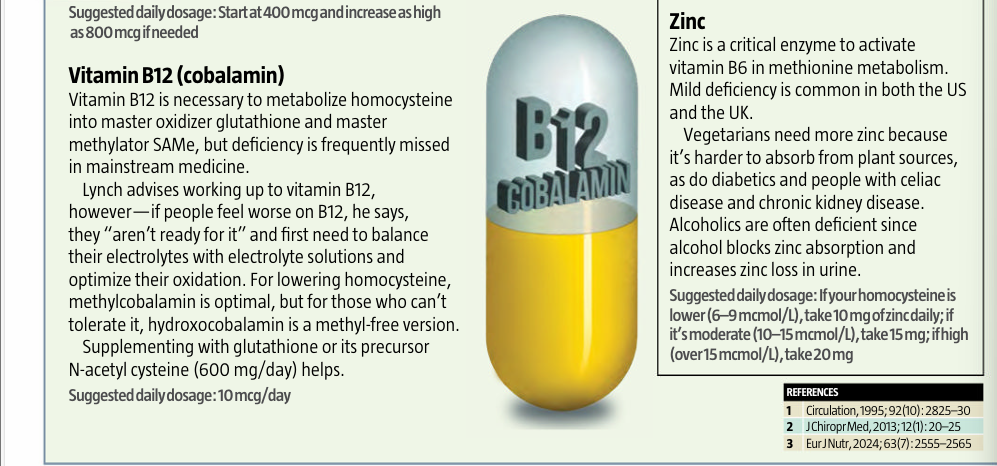
B vitamin
deficiencies High homocysteine almost always occurs in tandem with
low levels of vitamins B2, B6, B9 and B12.1 Magnesium
deficiency Without magnesium, it’s harder for the body to convert
homocysteine into glutathione or SAMe. Zinc deficiency Like magnesium,
zinc is needed to convert homocysteine to other molecules. Trimethylglycine
(betaine) deficiency This molecule is also used to remethylate
homocysteine, converting it to SAMe. Vegetarian/vegan
diet A plant-only diet risks methionine and vitamin B12
deficiency. Carnivore diet A strict carnivore
diet may lead to high methionine intake and low vitamin B9. Low stomach acid
or impaired digestion If you are unable to digest your food well to extract
nutrients like methionine, or if you can’t absorb B vitamins, your intake may
not be absorbed, and the effect is like deficiency. High alcohol
consumption Excessive alcohol use may block absorption and can lead
to deficiencies in vitamins B1 (thiamine), B6, B9 and B12 as well as zinc. In
alcoholics, higher homocysteine is related to alcohol cravings, withdrawal
seizures and increased dependency in a deadly feedback loop that spikes other
health risks as well.2 REFERENCES 1 Clin Chem, 2003; 49(2): 295–302; JAMA,
1993; 270(22): 2693–8; Am J Epidemiol, 1996; 143(9): 845–59 2 Clin Nutr, 2018; 37(3): 1061–1065; Biomedicines, 2020; 9(1): 7
3 Clin Chem Lab
Med, 2011; 49(3): 479–83 |
Obesity People who are
obese have significantly higher homocysteine levels2 High coffee
consumption Drinking more than 3 cups of coffee a day raises
homocysteine, an effect that appears only partially related to its caffeine
content.5 Kidney
impairment High homocysteine impairs kidney function, accelerates
kidney disease and raises the risk of heart attack and stroke in people who
have kidney disease.6 MTHFR genetic
variation MTHFR is the gene that codes for
methylenetetrahydrofolate reductase (MTHFR), an enzyme important for
metabolizing folate. In Europe and North America, 10–15 percent of the
population has a variant that significantly interferes with methylation of B
vitamins, and about 40 percent carry a variant but aren’t greatly affected by
it. Some
prescription drugs Methotrexate, corticosteroids, arthritis drugs,
metformin, L-dopa (for Parkinson’s), fibric acid derivatives and
cholestyramine (for high cholesterol and cardiovascular disease),
theophylline (for asthma and other lung diseases) and phenytoin (for seizures)
may raise homocysteine sharply.7 Nitrous oxide
(laughing gas) Used to calm anxious patients, nitrous oxide can
“irreversibly oxidize” vitamin B12 and lead to serious problems, especially
for those with MTHFR mutations, as in the case of a child who died after
receiving nitrous oxide during dental treatment.8 4 J Evid Based
Med, 2021; 14(3): 208–217 5 Curr Pharm Des,
2023; 29(1): 30–36; Am J Clin Nutr, 2002; 76(6):
1244–8 6 Leenus Tafline AE, “Homocysteine
and Renal Disease,” May 20, 2024, icliniq.com 7 Drugs, 2002;
62(4); 605-616 8 NEngl J Med, 2003; 349: 45–50 |
|||
|
|
|
|||
With
the importance of B vitamins in homocysteine’s metabolism and its breakdown
into harmless building blocks recently elucidated, McCully proposed that
deficiencies of vitamins B6 (pyridoxine), B9 (folate) and B12 (cobalamin) allow
homocysteine, normally benign, to rise to toxic levels.
At
every turn, his research seemed to confirm his theory: When he injected rabbits
with homocysteine, they developed arteriosclerotic plaques in their coronary
arteries within weeks. The plaques were larger if the animals were also fed a
diet deficient in vitamin B6.
But
when he gave them these vitamins, the animals’ homocysteine levels plummeted,
sometimes within hours. Other researchers reported similar findings in baboons.
Then, in 1976, Australian researchers published the discovery that coronary
heart disease was linked to elevated blood homocysteine in humans, too.2
McCully’s
theory was unwelcome at Harvard and in mainstream medicine, however, which had
latched onto the cholesterol-heart hypothesis.
The
homocysteine theory also threatened a burgeoning industry around
cholesterol-lowering drugs—within a decade, these drugs launched as the biggest
blockbusters of all time and today still generate more than $30 billion a
year.3 But there was no commercial interest in cheap, readily available and unpatentable B
vitamin therapy for potential heart attack and stroke victims.
Under
a new chief at Harvard in the 1970s, McCully’s theory was ridiculed, his
funding dried up and he was moved to the basement. Eventually he lost his
tenure and was told not to return.
At
a new position at the Veterans Affairs Medical Center in Rhode Island, he
quietly continued his research while other labs around the world continued
looking at homocysteine, confirming its critical role in cardiovascular
disease.4
Vindicated
at last
Eventually,
almost two decades after McCully’s dismissal, homocysteine made its way back to
Harvard. Nearly 15,000 male physicians aged 40–84 years, with no prior heart
attack or stroke, gave plasma samples and were followed up for five years.
The
Physicians Health Study found that homocysteine was strongly correlated with
heart disease. Participants who had levels in the top 5 percent of the normal
range were three times more likely to have a heart attack.5
It
was a turning point for homocysteine research. And the story got bigger, too.
In a cohort study in Norway, about 5,000 men and women aged 65–67 was recruited
as part of a national cardiovascular screening program that followed them for
four years. Those with higher homocysteine didn’t just die of cardiovascular
disease more often, they died of all causes more often.
A
five-point rise in homocysteine level translated into a 49 percent increase in all-cause mortality, a 50 percent increase in
cardiovascular mortality, a 26 percent increase in cancer mortality and a 104
percent increase in death by other causes.
“These
results should encourage studies of [homocysteine] in a wider perspective than
one confined to cardiovascular disease,” the researchers of the Hordaland
Homocysteine Study concluded.6
As
links to killer cancer and Alzheimer’s were uncovered, homocysteine research
exploded. Meanwhile, a few randomized trials, such as the HOPE and NORVIT
trials, tested whether lowering homocysteine with supplementary B vitamins
would reduce the risk of vascular disease after an event, and the results were
disappointing.7
It
virtually became mainstream medical dogma that B vitamins “don’t work” for
vascular disease—or anything else, for that matter. Many researchers noted that
studies of previous heart attack and stroke sufferers were biased, however, and
that research should be done on those who hadn’t had a vascular event.
The
dissenters said the trials were too short and questioned the doses. They also
noted that the folic acid supplementation mandated in some countries in the
late 1990s obscured the vitamins’ benefits in the trials.
One
critique, titled “Homocysteine: Call Off the Funeral,” said the HOPE trial had
found supplementation did not prevent heart attacks but buried any mention of
its own data showing it did prevent strokes. There were just too many
unanswered questions about homocysteine to call it a dead end, its authors
reasoned.8
Fast-forward
another two decades, and many of those questions are still unanswered. During
this time, the cholesterol theory has gone virtually unchallenged by mainstream
medicine, and the lipid-lowering drug
bonanza
has continued.
Many
researchers, however, say that if homocysteine is central to even just 10–15
percent of vascular incidents, that’s too many to ignore when there are 4,400
heart attacks and strokes daily in the US alone. Add that to other diseases,
and at higher levels perhaps, and the homocysteine carnage looks enormous.
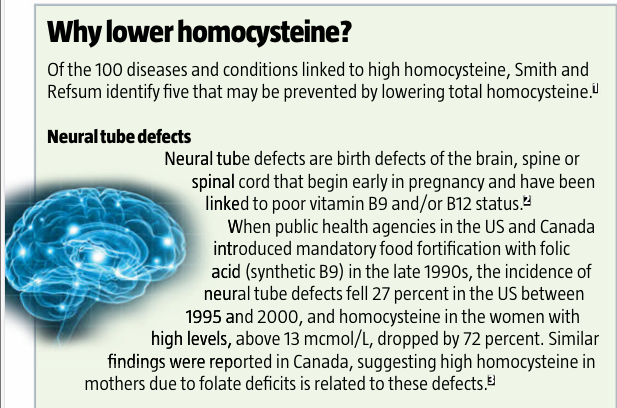
100
diseases
“We
have reviewed the literature and have identified more than 100 diseases or
conditions that are associated with raised concentrations of plasma total
homocysteine,” say Dr David Smith and Dr Helga
Refsum. Smith is emeritus professor of pharmacology at the University of
Oxford, and Refsum is a professor of nutrition at the University of Oslo in
Norway and a lead author of the Hordaland Homocysteine Study.
“The
commonest associations are with cardiovascular diseases and diseases of the
central nervous system, but a large number of developmental and age-related
conditions are also associated. Few other disease biomarkers have so many
associations.”
The
list of diseases and conditions that high homocysteine has been linked to reads
like a pathology text: alcohol abuse, Alzheimer’s, anxiety, autism, cardiovascular
disease, cancer, cognitive impairment, congenital defects, depression,
diabetes, gum disease, low birth weight, Parkinson’s disease, polycystic
ovarian syndrome, schizophrenia and more.9



Two
key pathways
Homocysteine
sits at the center of two key biochemical pathways in the body:
oxidation-reduction (redox) and methylation. The body constantly produces free
radicals as byproducts of normal reactions, and it makes even more when we
exercise too much, eat bad oils or burned food, get a sunburn, breathe dirty
air or live with an inflammatory disease.
This
oxidation process, which is at the heart of aging, is countered by
antioxidants. The body’s master anti-aging antioxidant, glutathione, is low
when homocysteine is high—something is choking the system that converts
homocysteine into glutathione, which is needed for detoxification and
oxidation, so homocysteine builds up. Like high homocysteine, low glutathione
is linked to death from all causes.
Methylation
is another key chemical process that happens billions of times a minute as our
body does things like break down nutrients into usable molecules, convert
neurotransmitters or hormones, or detox poisons from food or the environment.
The donation of a methyl molecule (made of one carbon and three hydrogen
atoms), called methylation, happens in all these processes. Methylation is used
to repair broken DNA and switch genes on and off, including those in many
cancers.
“Homocysteine
rises if you’re not doing methylation properly,” says Patrick Holford, author
of The Homocysteine Solution with Dr James Braly (Piatkis Books, 2012).
Methionine,
which we consume in protein-rich foods like meat and fish, is methylated to
become homocysteine. That in turn is either converted into glutathione or
remethylated and turned into the body’s most important methyl donor molecule,
S-adenosyl-methionine (SAMe) which fuels myriad other major methylation
reactions.
This
biochemistry all depends on levels of B vitamins as well as nutrients like zinc
and magnesium that catalyze the conversions. If those nutrients are in scant
supply, homocysteine builds up and begins its wrecking cascade, increasing free
radicals, stiffening blood vessels, triggering inflammatory pathways10 and
mitochondrial dysfunction,11 and damaging proteins,12 the blood-brain barrier13
and DNA.14

